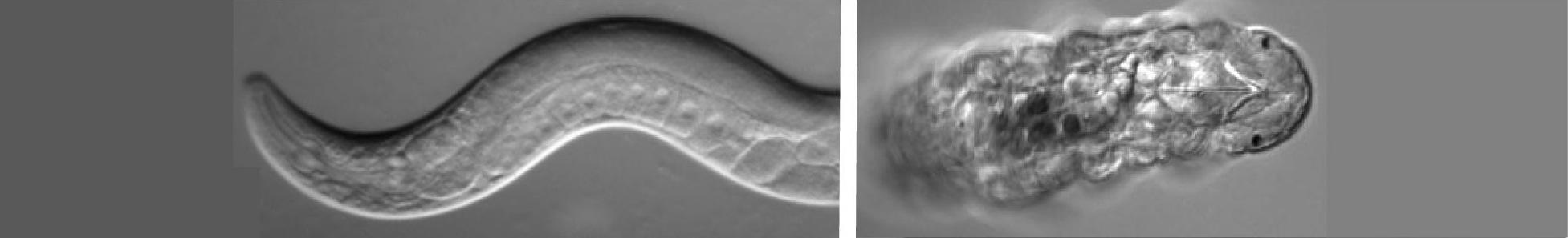
Caenorhabditis elegans Resources
Introduction
I created this page as part of my involvement in the Science Education Partnership program at Fred Hutchinson Cancer Research Center while I was a grad student in Jim Priess's Lab. Every summer, nearly two dozen teachers are invited to FHCRC for an intensive 13-day session. Nearly half of the session is spent in the SEP Teaching Lab, where teachers practice basic techniques in molecular biology (pipetting, PCR, gel electrophoresis, etc.) and learn about resources available to biology teachers. Teachers complete a research project under the guidance of a scientist mentor at one of several host sites. In addition to helping teachers with their project, the graduate-student mentors prepare a seminar series on a topic of interest. The following information comes from our seminar series, "Model Organisms.
Any questions, comments, broken links, etc.? Feel free to e-mail me ().
Contents
- Background Information and References
- What can worms teach us? Profiles of scientists and their research
- C. elegans in the News
- Setting up for your worm lab
- Applications in the Classroom: Lessons and Ideas
Background Information, Databases and Resources
- The primary source of public-access information about C. elegans. Includes detailed descriptions of all known C. elegans gene sequences, genotypes, phenotypes, mutations, etc.
- An invaluable resource that includes reviews on all aspects of worm biology, experimental methods, and resources. Written by key researchers in the field, updated regularly, and freely accessible. An excellent source of background materials.
- An informal newsletter for the worm community, where interesting observations and data, new techniques, and other information are shared. The archives are a treasure-trove for those interested in learning about the history of C. elegans research from the trenches.
- Handbook of C. elegans anatomy, worm images and diagrams, movies, and many other resources.
WormClassroom
- Many resources for educators interested in bringing C. elegans into the classroom. Includes a history of worms as a model organism, examples of contributions of C. elegans to research, and lots of examples of lesson plans and strategies for incorporating worms into science classes. (WormClassroom no longer appears to be maintained, but older content can still be accessed via the Internet Archives.)
Caenorhabditis Genetics Center
- This center is the main supplier of worm strains to the C. elegans community. Whenever mutant strains are generated, they are sent to the CGC, where they can then be provided to other researchers and educational institutions upon request.
- A collection of digital videos of diverse phenotypes in wild-type and mutant worms (maintained by Dr. Bob Goldstein, UNC-Chapel Hill).
- A service of the National Library of Medicine (a division of the National Institutes of Health); a number of biology textbooks available online.
- Scroll down to "C. elegans II" - the 1997 book that contains detailed descriptions and reviews about the worm.
- Comprehensive, searchable database of research papers that reference C. elegans
NEMAPLEX: The Nematode-Plant Expert Information System
- A Virtual Encyclopedia on Soil and Plant Nematodes
Model Organism Week: Getting to Know Your Worms (C. elegans)
- Blog post by Jessie Geahlen for the benchfly site. Check out the 19 "Reasons why C. elegans are great model organisms"!
The tiny worm with the big impact
- Blog post by Katie Pieper for the Genetics Society of America. Accessible overview of the contributions of C. elegans to genetics research.
Society for Developmental Biology Education Links
- Nice collection of educational resources available online for all grade levels. Resources include not only C. elegans but other model systems as well.
What can worms teach us? Profiles of scientists and their research
Sydney Brenner
- Profile of Sydney Brenner, including the letter he wrote to Max Perutz, describing his choice of C. elegans as a model organism with which to address major biological questions. (This page was originally maintained by Leon Avery on the C. elegans server. The website is no longer maintained, but is accessible via the Internet Archives.
Web of Stories: Sydney Brenner
- An interview with Sydney Brenner, who shares his life and research. His discussion of the process that led him to pursue research on C. elegans begins with video #126.
- A collection of biographies, perspectives and tributes to Sydney Brenner, who died 5 Apr 2019 at age 92.
- Profile of Ellsworth C. Dougherty, who influenced Sydney Brenner's choice of model organisms.
Cynthia Kenyon: 'The idea that ageing was subject to control was completely unexpected'
- This interview, conducted in March 2013 by Catherine de Lange for The Guardian, focuses on Dr. Cynthia Kenyon, who uses C. elegans as a model organism to identify genes involved in aging
- Inspiring profile of Dr. Lois Edgar, who studies the development of C. elegans embryos, and who took a non-traditional path to pursue a career as a scientist.
Brain Science Podcast: Guy Caldwell
- Interview with Guy Caldwell, PhD, a researcher at the University of Alabama who studies C. elegans to better understand the basis of movement disorders, such as Parkinson's Disease.
A Conversation with Cori Bargmann Puts Her Mind to How the Brain Works
- This interview, conducted in September 2015 by Claudia Dreifus for the New York Times, profiles Cori Bargmann, a neurobiologist at Rockefeller University, who uses C. elegans as a model for understanding the development and function of neurons.
- Learn more about why three prominent worm researchers (Sydney Brenner, H. Robert Horvitz and John Sulston) were awarded the 2002 Nobel Prize in Physiology or Medicine.
Worms contribute to another Nobel Prize!!
- Andrew Z. Fire and Craig C. Mello were awarded the Nobel Prize in Physiology or Medicine in 2006, for their research in RNA interference, or gene silencing by double-stranded RNA, in C. elegans.
More Nobel recognition for the worm!!!
- Martin Chalfie (along with Roger Tsien and Osamu Shimomura) was awared the Nobel Prize in Chemistry in 2008, for his role in developing Green Fluorescent Protein as a valuable tool for studying protein expression and dynamics in living worms.
An Interview with Victor Ambros, David Baulcombe, and Gary Ruvkun
- Victor Ambros, David Baulcombe, and Gary Ruvkun were awarded the 2008 Albert Lasker Basic Medical Research Award for their discovery of microRNAs. microRNAs were originally discovered in C. elegans and are now known to play important roles in gene regulation in many diverse organisms.
Worms, Longetivity and Diabetes
- An article written by Sean Henahan about the discovery of a longetivity gene in worms, and its implications for human biology (part of the Access Excellence collection)
C. elegans in the News and Press
Science . Vol. 282, Number 5396, Dec. 11, 1998.
- Special issue reporting the completion of the C. elegans genome.
- Several good review articles - of particular interest is Mark Blaxter's review, "Caenorhabditis elegans Is a Nematode" (pg. 2041-2046).
Barry, Dave. "Here's the complete poop on mutant Seattle worms." Seattle Times. Seattle, Wash.; Mar 21, 1994. pg. B4.
- A somewhat off-beat profile of Dr. Jim Thomas's lab (at the U. Washington), which studied defecation in worms (among other things)
BBC News. "Alzheimer's gene 'diabetes link'". BBC News. June 14, 2012.
- Report on research in C. elegans that provides evidence that mutations in genes linked to Alzheimer's Disease is also involved in insulin metabolism, suggesting that Alzheimer's Disease and diabetes may be linked in humans.
BBC News. "Worm lifetime 'longer in space'". BBC News. July 6, 2012.
- Report on new findings that C. elegans worms living aboard the International Space Station, then returned to earth, had accumulated mutations in several genes already known to confer longer lifespans in C. elegans.
Cooke, Robert. "Worm's complex genome unraveled; 'Map' is big step forward for scientists." Seattle Times. Seattle, Wash.; Dec. 11, 1998. Pg. A3.
Donn, Jeff. "Enzyme that helps extend life is discovered." Seattle Time. Seattle, Wash.; May 13, 1999. Pg. A6.
Elias, Paul. "Pursuing Healthier Bacon Through Genetic Engineering and Cloning." March 26, 2006. Associated Press. [This article appeared in the Seattle Post-Intelligencer as Worm DNA gives bacon a heart-healthy boost, March 27, 2006, pg. A5.]
Grady, Denise. "A Worm's Life: Right Mutation Makes It Long but Very Dull." New York Times. New York; May 21, 1996. pg. C1.
- Describes the discovery of "clock" genes, which affect life-span (called "clock" genes due to their role in regulating the length of the cell cycle)
Huntington, Rebecca. "Tiny worms may offer clues to cures for human diseases." Seattle Times. Seattle, Wash.; Jul 25, 1999. pg. B3.
- Profile of Bruce Bowerman, a C. elegans researcher at U. Oregon, who was once a post-doctoral fellow in Jim Priess's lab at Fred Hutchinson Cancer Research Center
Scicurious. "It's all about sex: the connectome of a C. elegans male." The Scicurious Brain (Scientific American Blog). Posted online Aug. 6, 2012.
- Nice discussion of recently-published research that maps the neural networks in C. elegans males. One of the key findings of this research is that the vast majority of male neurons are dedicated to mating.
- See also The Mind of a Male?, another perspective on this research, written by Dmitri B. Chklovskil and Cornelia L. Bargmann, and published in Science's July 27, 2012 issue (volume 337, pp. 416-417). There is also a link to the original research article. (A subscription is required to read the full Perspective.)
Steenhuysen, Julie. "Genes switch altered sex orientation of worms." Reuters. Published online Oct 26, 2007.
Swaminathan, Nikhil. "The skinny on fat: you're not always what you eat." Scientific American. Published online June 4, 2008.
- Describes a recent study from Kaveh Ashrafi's lab showing that serotonin has separate effects on feeding rate and fat storage in C. elegans.
Sulston, John and Georgina Ferry. The Common Thread: A Story of Science, Politics, Ethics, and the Human Genome. Joseph Henry Press, 2002. This book provides Sulston's account of the development of the Human Genome Project, starting from the C. elegans sequencing project.
Wade, Nicholas. "The Four-Letter Alphabet That Spells Life." New York Times. New York, N.Y.; Jul 2, 2000. pg 4.4.
- Describes research at UCSF demonstrating that a single gene controls both social and solitary feeding in worms
Wade, Nicholas. "An Adroit Director Of an Unwieldy Team." New York Times. New York, N.Y.; Jun 27, 2000. pg. F.3.
- Short biographical information on the key players in the worm genome sequencing project
Wade, Nicholas. "Animal's Genetic Program Decoded, in a Science First." New York Times. New York; Dec 11, 1998. pg. A1.
Back to TopSetting Up for Your Worm Lab
Obtaining worms
The standard recipe for preparing Nematode Growth Medium (NGM) is included in Theresa Stiernagle's Worm Maintenance chapter in WormBook (Section 3.2). The entire chapter is a valuable resource for growing and maintaining worms. All reagents and consumables are readily available through most scientific-supply companies. 60 mm x 15 mm disposable petri dishes are standard for worm maintenance.
Carolina Science and Math Catalog provides a nematode "kit" that includes pre-made Nematode Growth Agar, bacteria, and petri dishes. The components can be ordered as a kit (such as catalog # 211390 or #173525), or individually:
- Caenorhabditis elegans N2 — catalog # 173500
- Nematode Growth Agar: catalog # 173520
- E. coli strain K-12: catalog # 155068
- 60 mm x 15 mm disposable petri dishes: catalog # 741246
While plates are not difficult to prepare, they can be time-consuming and pricey, depending on the number of plates needed and cost of consumables. I have had good experiences with LabExpress, which will prepare and ship several different types of worm plates.
The Caenorhabditis Genetics Center will provide worm strains and bacteria for a small fee, although exceptions can be made for teachers in secondary schools upon written request (see their website for details). The preferred bacteria for C. elegans is E. coli strain OP-50, which is a slower-growing strain than K-12. The CGC will provide OP-50 upon request. The one caveat is that the CGC is primarily a two-person operation, and orders can sometimes take up to 3 - 4 weeks to process. Therefore, it is necessary to plan ahead and allow plenty of time when ordering through the CGC. (They will not provide agar, so you would still need to buy this through another source).
I can also provide some strains to teachers in the Science Education Partnership program through Fred Hutchinson Cancer Research Center - see this page for more information.
Obtaining mutants:
Mutant worms with obvious phenotypes are not only useful in studying Mendelian inheritance or genetic mapping, but they are also just plain cool to look at. Here is a very short list of the more visually-interesting mutants:
- dpy-5 and dpy-6: these worms are "dumpy" - very small (some look like mini-footballs). These are excellent for genetic studies (such as studying Mendelian inheritance or mapping a mutant) since they’re located on separate chromosomes, and have distinct phenotypes.
- rol-6 : these mutants are "rollers" - because of a defect in their cuticle, they are constantly rolling around on the plate (they are "right-hand rollers", meaning they roll in a clockwise direction). It is a bit difficult to detect at first, but once the phenotype is recognized, it is very easy to pick out.
- unc-4 : "unc" means "uncoordinated"; these worms are able to move forward, but cannot move backward (this is evident by tapping worms on their heads - wild-type worms will move backward 1 - 3 body lengths in response to head-tapping; unc-4 worms will not).
- unc-22 : these worms are called "twitchers" - they constantly twitch/shake. This phenotype is caused by a mutation in a gene that encodes a fibronectin-type protein, which has been implicated in several human neurological disorders.
- unc-119: another neurological defect; worms are paralyzed, and small ("dumpy-ish")
Handling worms:
Worms are not terribly fussy about their living conditions, as long as they have a plate of bacteria to eat!
The wild-type strain of C. elegans is "N2" (these are descendants of a strain originally isolated in Bristol, England, in the 1940s). Worms can be maintained at room temperature. (If you have the ability to control for temperature, such as an incubator, 20°C is the ideal incubation temperature — at this temperature, it takes about 2.5 - 3 days from hatching to adulthood. At 15°C, it takes an extra day. At 25°C, it is about 1/3 as fast.) A plate of starved-out worms, wrapped in parafilm, will be viable for several months if kept at 15°C.
Aseptic techniques must be observed at all times! Be careful to minimize the exposure of plates to air, since fungi or airborne microbes can contaiminate a plate. While these contaminants are usually not harmful to worms, they are an inconvenience to worm researchers.
To maintain well-fed worms:
Day 1: Pour plates — prepare nematode growth medium as directed, and dispense into 60mm x 15mm plates (about 1/2 full). Allow plates to cool and solidify overnight. (Plates can be prepared up to 1 month ahead of time, and kept at 4°C. Pull plates out the day before needed and warm to room temperature). Prepare E. coli culture as directed.
Day 2: Seed plates — once plates are at room temperature, add 300 Ál of E. coli to the center of the plate. (If you don’t have a micropipetter, you could also use a Pasteur pipette or eye dropper — about 3-4 drops of culture. There should be a pool of liquid in the center of the plate, about half the total diameter of the plate). Be careful not to swirl the plates too much — most of the bacterial should be concentrated in the center, surrounded by a ring of bacteria-free agar. Allow plates to sit overnight at room temperature. Unused plates can be stored at 4°C for up to a month. Be sure to warm plates to room temperatures before placing worms on plate.
Day 3: Place worms on plate — worms can be transferred in one of two ways:
a. "Chunking" — using a sterile scapel, cut a small cube out of a plate of worms — about 1cm x 1cm. Cut from an area of the plate where there is a medium density of worms (not too high, not too low). Place the chunk, worm-side down, onto the freshly-seeded plates. Chunking will result in a plate of mixed-stage worms, which is useful for observing the continuum of C. elegans development from embryo to adult.
b. "Picking" — using a tool called a pick, made out of platinum wire melded to the end of a glass Pasteur pipet (platinum is used since it cools faster). Picking is used when you want to control for the age of the worm, ensure that all progeny on a plate are from the same hermaphrodite, or to transfer worms from a contaminated plate to a fresh plate. Platinum wire can be obtained from Sigma-Aldrich (catalog #357359) or Tritech Research (catalog #PT-9901). [Nancy Hutchison had the great idea to repurpose platinum wire from old gel boxes that are no longer being used for electrophoresis. I'd just double-check to make sure there is no ethidium bromide contamination on the wire, but otherwise, this would definitely save some money.]
note: Picking can be a challenge for some students. [I made this video to help my students get a feel for how I pick worms.] I strongly encourage students to keep trying - they will get it eventually. However, a possible alternative is to use disposable microcapillary pipets (20 - 25 Ál capacity) - draw the pipet over a flame and pull it apart to make two micropipets. Create a bore by lightly pressing the tip of a pipet against the pad of a finger, or by pinching with your fingernails. You want a bore that is big enough to draw up individual worms of the desired stage (but not too big). The Kimble brand includes a rubber bulb with a micropipet adapter (Kimble item #:71900-20, available from Fisher and other suppliers). Attach the pipet to the bulb, and draw up some liquid (a saline solution such as M9 is preferred, but distilled water works, too). While looking through the microscope, place several drops of liquid on top of the worm (enough that the worm is swimming around in the liquid), leaving some liquid in the pipet tip. Suck up the worm, then transfer to a new plate.
Day 4: Continue to check your plates daily. If any plates appear to be "starving out" (most of the bacteria has been eaten), then use the chunking method to transfer worms to a new plate. Save one starved plate as a "stock plate" to use in case you have contaminants, or your N2 strains start displaying weird phenotypes as the result of spontaneous mutations. I try to replace my stock plates every 6-8 weeks.
How to handle contaminants:
Fungal: Easiest to handle — if fungi develop on a plate, use a pick to remove worms to a new plate. Transfer worms to new plates for several days, until it is clear that no fungal spores were transferred with the worms. (Save the contaminated plates for your students — they are incredibly cool to look at under a dissecting scope! Be sure to seal the plates in parafilm first, to prevent fungal spores from escaping).
Bacterial: Sometimes, other bacteria or yeast will colonize a plate. These are easy to detect since they will be a different color than the E. coli. If there are contaminated plates, then there is a concern that worms have ingested the contaminating bacteria, and therefore will contaminate any new plates they are moved to. If you have a stock plate, then toss the contaminated plate (wrapped in parafilm), and chunk out worms from the stock plate. If not, then to remove this contamination, prepare a solution of 50% bleach and 50% 1M NaOH (sodium hydroxide). Place one drop of the bleach solution on the unseeded portion of a fresh plate. Use a pick to transfer several adults from the contaminated plate (the adults should have plenty of eggs in their uterus). The bleach will dissolve the adults (and kill any bacteria). The eggs will hatch normally, since bleach cannot penetrate their egg shell. The next day, transfer the hatched larvae to a fresh plate.
Getting Help:
Several labs in the Seattle area work with C. elegans, and the majority of these labs have at least one grad student who has been involved with the SEP program, and would be willing to help teachers. The best way to get in touch with these labs is through the SEP program. WormBase maintains a searchable list of worm labs - you can search for specific lab heads, or find all labs in a specific location.
Back to TopApplications in the Classroom: Lessons and Ideas
- NSF-funded resource sponsored by the DNA Learning Center at Cold Spring Harbor Laboratory
- Series of experiments using RNA interference to disrupt gene expression in C. elegans
U. of Washington Genome Sciences Education Outreach
- "Genes, the Environment, and Me" module allows students to explore how mutant C. elegans strains respond to environmental stress.
"Worms are a lot more similar to people than you may think!" (The original link was taken down after Sam Ward's retirement, but this link points to an archived version hosted by the Internet Archives.)
- A simple-but-effective introduction to the study of homologous genes in different organisms
- Online exercise that demonstrates how sequence databases (such as GenBank) can be used to compare gene and protein sequences from different organisms
Worm Breeding for Super Geniuses
- An excellent guide to performing crosses and mapping mutations in C. elegans, written by David S. Fay, Dan Starr, Andy Spencer, and Wade Johnson.
BrainU - "Learning Neuroscience Through Inquiry Pedagogy" - resources for teachers in grades 5 - 8, led by Dr. Janet Dubinsky, Dept. of Neuroscience at the University of Minnesota. Several C. elegans-specific labs are available, including:
- Caeno-WHAT?? - lab activities designed to investigate C. elegans taste and smell preferences; geared to students in grades 5-8.
- Chemotaxis using C. elegans - geared to students in grades 5-8.
- C. elegans and Alcohol - investigation of the effects of alcohol on C. elegans. For middle and high school students.
Understanding the Uncs! - a series of activities for high school students designed by Dr. Brock Grill at The Scripps Research Institute, Florida.
Alcohol, C. elegans and You" - Drug Abuse Research Teams (D.A.R.T.) (The original link is no longer active. This link points to an archived version hosted by the Internet Archives.)
- Describes how to set up and test C. elegans for effects of exposure to drugs and alcohol in the classroom. This website describes the DART research projects at Abraham Lincoln High School in San Francisco, CA.
The following articles describe methods of using C. elegans in the classroom, and were published in Life Sciences Education, an open-access journal for educators at all levels.
- Analyzing Defects in the Caenorhabditis elegans Nervous System Using Organismal and Cell Biological Approaches (M. Guziewicz, T. Vitullo, B. Simmons and R. E. Kohn, Ursinus College, 2002).
- Identifying Novel Helix-Loop-Helix Genes in Caenorhabditis elegans through a Classroom Demonstration of Functional Genomics (V. Griffin, T. McMiller, E. Jones and C. M. Johnson, Morgan State University, 2003)
- Student Learning of Early Embryonic Development via the Utilization of Research Resources from the Nematode Caenorhabditis elegans (F-M. Lu, K. W. Eliceiri, J. M. Squirrell, J. G. White and J. Stewart, University of Wisconsin-Madison, 2008).
More suggestions for Using C. elegans in the Classroom
- Observations/descriptions: Describe characteristics of wild-type worms — identify different stages of the life cycle.
- Observation/description: Mitosis is readily observed by observing eggs under a compound microscope. Directions: Make up 2% agar solution (0.2 g agarose in 10 ml sterile water; heat until boiling). Using an eyedropper, place one drop of agar solution in the middle of a microscope slide. Immediately place another microscope slide over the drop, perpendicular to the first slide. Let cool. Using a sterile scapel blade, cut open several adults filled with eggs ("gravid" adults) in a watch glass filled with M9 or S basal media (see "C. elegans 101" for recipes). Use a micropipetter to transfer eggs from the watch glass onto the agar pad. Place a drop of M9 or S basal media on a cover slip, and invert over the microscope slide. Eggs should be happy for several hours. Several divisions can be observed within a class period. [AP level: Since cell division is asymmetric in C. elegans embryos, have students speculate what could cause asymmetric division, and what impact such division could have on the embryo. In a nutshell, asymmetric division results from the repositioning of the spindle poles after each division.]
- Observations/comparisons: Describe locomotion in wild-type and several locomotion-defective mutants. Compare and contrast between different strains, and between different life-cycle stages. (For example, compare how different worms behave in response to being tapped on the head. The unc-4 mutant adults are unresponsive. However, mutant larvae are more responsive.) [Special Note: A simple tool for touching the heads and tails of worms is to mount an eyelash at the end of a wooden toothpick. Either glue or clear nail polish work equally well as an adhesive]
- Testing a hypothesis/designing experiments: Chemotaxis response in worms. Wild-type worms have a well-developed olfactory system, and are strongly attracted to some substances, and strongly repelled by other substances. Students can use a chemotaxis assay to test different compounds. For example, wild-type worms are strongly attracted to sodium ions (such as in NaCl). Several chemotaxis-defective mutants (such as che-1) are not attracted to sodium ions.
- Mendelian inheritance: since worms are self-fertilizing, strains which are heterozygous for a specific mutation can be used to test Mendelian ratios. The fact that one worm can lay up to 300 eggs gives a large enough sample size for expected Mendelian ratios to be realized (especially when class data are pooled). In addition (or instead of), males can be mated to a strain homozygous for a mutation, and expected/observed ratios calculated.
- Genetic mapping (AP level): worms have 6 chromosomes, each with well-defined physical and genetic maps. Use two-factor crosses to determine on which chromosome a particular mutation is located. (Have six groups of students performing crosses with six markers, one on each chromosome. Then pool data to determine the correct chromosome). Three-factor crosses are probably too labor-intensive and time-consuming for a high-school class, but you could discuss with students how they would pinpoint a more accurate location for the mutation.
- RNA interference: a reverse genetics approach to disrupt gene function. RNAi is well-established technique for specifically knocking down known genes in order to study their function in C. elegans. Double-stranded RNA (dsRNA) is synthesized, then introduced into worms by microinjection, soaking worms in a solution containing concentrated dsRNA, or by feeding worms bacteria that express dsRNA upon induction of a T7 RNA polymerase. Soaking or feeding are the most commonly used methods in classroom worm labs. Julie Ahringer's Reverse Genetics chapter in WormBook provides an introduction to RNAi, and provides detailed instruction on setting up an RNAi-by-feeding experiment. The Silencing Genomes site, sponsored by the DNA Learning Center at Cold Spring Harbor Laboratory is an excellent resource for those planning to do RNAi experiments in their classes. The site describes the principle of RNAi, and lists available bacteria strains that produce dsRNA for different genes.
- Bioinformatics application: use tools such as "Online Mendelian Inheritance in Man" (OMIM) to have students research the genetic mutation that causes a disease/disorder/syndrome of interest (have students select from a list of disorders caused by single-gene mutations). OMIM should have a link to the gene sequence as part of the description. Have students locate the gene sequence, then use the BLAST server to identify homologous genes in other organisms. If you want to focus on a single organism (such as C. elegans), use the C.elegans- specific BLAST server at WormBase. Use Wormbase to identify the function of this gene in worms. Another easy way to access sequence databases is through "Sequence Server" which is part of the Dolan DNA Learning Center collection. [The objective of this exercise is to help students understand that many genes are conserved between a variety of organisms. Since the function of genes is often difficult to study in humans, having model organisms such as C. elegans allows us to study a particular gene, then make predictions about the functions of homologous genes in humans].